How CMMS supports compliance across facilities, assets, and audits
In this post
%20(1).avif)
1
2
3
This guide explains how CMMS enables compliance by industry. Explore features to evaluate and best practices for audit readiness in 2026.
Compliance now carries real operational and career risk for facility, maintenance, and compliance leaders. Missed inspections, undocumented repairs, and scattered records surface fast during audits, placing teams under pressure to defend decisions made months earlier.
As regulatory scrutiny increases across healthcare, manufacturing, food processing, energy, and other critical industries, accountability no longer stops at completing the work itself. Auditors expect proof that work followed defined standards every time.
Industry research backs this shift. Regulatory compliance is a major factor driving CMMS (Computerized Maintenance Management Software) adoption in the U.S., especially in healthcare, pharmaceuticals, food processing, and energy industries (Grand View Research). Teams no longer adopt systems for convenience. They adopt them to protect operations when regulators arrive.
A well-implemented CMMS platform gives teams the structure to treat compliance as part of daily operations rather than a scramble before inspections:
- Preventive maintenance schedules document required tasks. Inspections capture safety and quality checks in real time.
- Work orders connect issues to corrective actions.
- Reporting pulls that history into audit-ready records that stand up under review.
That foundation makes compliance repeatable, provable, and defensible across every facility, asset, and audit cycle.
What CMMS compliance really means for facilities and maintenance teams
CMMS compliance means using a maintenance management system to consistently document, track, and prove that required maintenance, inspections, and corrective actions were completed according to defined standards. During audits, regulatory bodies assess how well teams follow those standards in daily operations, not how quickly they react once problems surface. Clear, complete records demonstrate how work progressed from discovery to resolution across the full asset lifecycle.
Compliance does not stop at task completion. A CMMS must preserve when work occurred, who approved it, and how it aligned with standard operating procedures so teams can confidently defend maintenance decisions during regulatory reviews.
Core CMMS capabilities make that defensibility possible on a daily basis:
- Compliance tracking and asset records capture asset data, conditions, approvals, and changes at every step.
- Preventive and predictive maintenance programs structure recurring work and surface risk before failures disrupt operations.
- Work order management workflows ensure inspection findings and issues are always linked to documented corrective actions.
- Reporting and dashboards consolidate maintenance and inspection data into audit-ready compliance reports without manual reconciliation.
Audit documents teams pull first
Most audits begin with a short list of records. Maintenance teams usually need preventive maintenance history for regulated equipment, lists of overdue inspections, corrective action logs tied to recent findings, and asset condition reports that reflect how maintenance decisions align with regulatory standards. A CMMS that produces these compliance reports in minutes shortens every audit conversation.
Each audit record auditors request is produced by a specific CMMS capability. The table below connects those capabilities to compliance outcomes.
CMMS compliance features buyers should evaluate
Not all CMMS platforms handle compliance the same way. Focus on the features that shape audit readiness and reduce compliance exposure at scale.
- Audit-ready reporting: Pull filtered records for a defined asset class across a selected date range, then export compliance reports when regulatory bodies request maintenance history.
- Role-based access control: Restrict editing rights, track approvals, and protect asset data so audit trails reflect real accountability.
- Workflow configurability: Align inspection, maintenance, and corrective action workflows to regulatory requirements and standard operating procedures.
- Multi-site consistency: Apply the same maintenance management and inspection standards across locations while preserving local accountability.
- Asset history and traceability: Retain long-term records that connect findings, actions, asset conditions, and outcomes for regulated equipment.
- Integration readiness: Connect the CMMS to monitoring, calibration, or predictive maintenance systems that support regulated workflows and deliver real-time insights.
Facilities teams usually compare platforms based on how well each system protects them during audits, once regulatory pressure comes into play. The best CMMS software stands out when compliance remains intact even as operations scale.
CMMS compliance by industry
Compliance requirements look different depending on what teams manage and who audits their work. A strong CMMS adapts to each regulatory environment while relying on a common maintenance foundation across all facilities.
Healthcare teams using a healthcare CMMS track patient safety checks, equipment maintenance, and corrective actions required for accreditation. Districts and campus leaders working with a CMMS for education centralize inspections and asset history across aging buildings. Manufacturing, food production, and energy operations apply the same structure to meet safety, sanitation, and environmental standards.
The table below highlights how compliance expectations differ by industry and how a CMMS supports audit readiness across each regulatory environment.
When to expand beyond CMMS for compliance
Many organizations meet compliance obligations with a well-implemented CMMS that supports asset tracking, safety inspections, and audit reporting. Operations with growing asset portfolios, multi-site complexity, or tighter regulatory scrutiny often need added systems that extend compliance coverage without replacing the CMMS foundation.
Enterprise asset management
Large organizations manage layered asset hierarchies, capital plans, and multi-year audit histories tied to asset lifecycle performance. Enterprise asset management software extends asset tracking across facilities and supports long-term decision-making informed by manufacturer guidelines, ISO standards, and internal governance requirements.
Teams evaluating CMMS vs EAM, or reviewing the best enterprise asset management software, often reach this stage after compliance reviews expose gaps tied to scale rather than execution.
Alarm monitoring
High-risk environments require documented responses to safety and operational alerts tied to safety inspections. Alarm monitoring software connects real-time system events to maintenance actions so every alert produces a tracked work order and audit trail. That visibility protects teams when regulatory bodies review how issues surfaced and how fast corrective action followed.
Calibration management
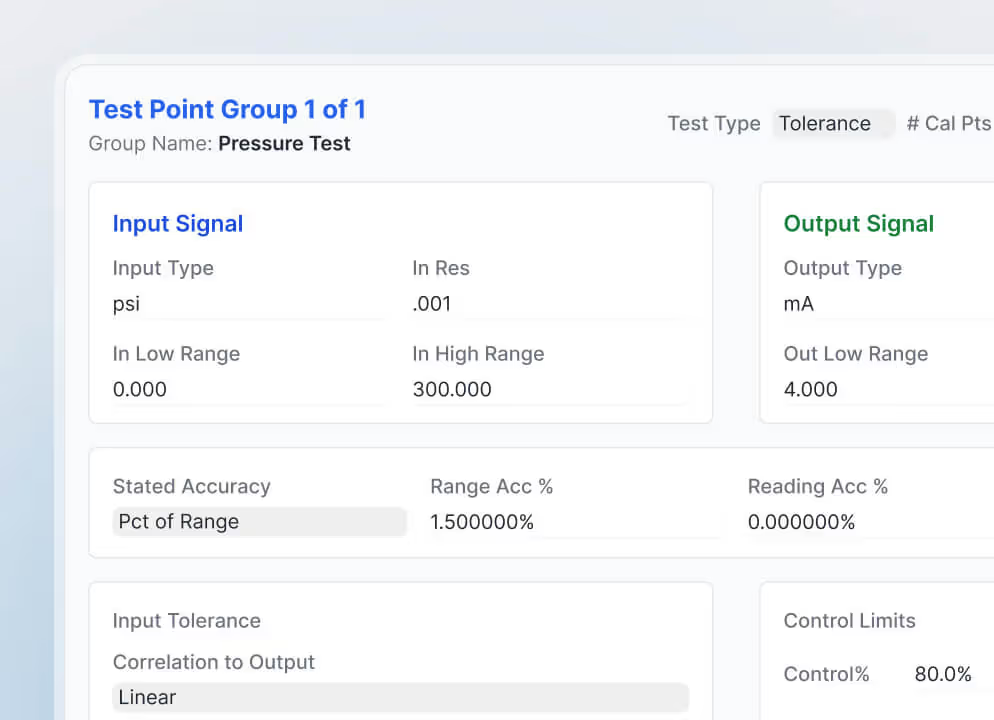
Regulated instruments demand proof of accuracy, tolerance, and traceability. In healthcare and life sciences, FDA regulations governing medical devices often require calibration evidence beyond standard maintenance logs.
Calibration management software captures schedules, results, digital signatures, and historical records tied to manufacturer guidelines. Without dedicated calibration records, CMMS data leaves quality auditors with unanswered questions.
CMMS best practices to support ongoing compliance
Getting compliance-ready requires disciplined daily processes supported through maintenance management software rather than last-minute reporting.
- Standardize preventive maintenance schedules: Assign PM intervals at the asset level and review overdue rates weekly. Rising overdue counts often surface before regulatory agencies flag a compliance violation.
- Use inspection templates tied to safety protocols. Track inspection completion rates each month and align forms with the regulatory frameworks that govern your industry. Gaps in completion data often signal exposure during regulatory reviews.
- Link every issue to a corrective work order: Require work orders for all inspection findings and safety issues so electronic records show how problems moved from discovery to resolution.
- Review compliance dashboards routinely: Scan open inspections, late PMs, and missing documentation each week. Real-time visibility shortens response time when regulators request proof.
- Protect data integrity with role controls: Restrict editing rights, require digital signature approval for regulated actions, and preserve audit trails inside electronic records. Clean documentation protects teams when compliance questions surface.
Bringing it together: A practical approach to CMMS compliance
Many organizations meet their compliance obligations with a well-implemented CMMS when requirements remain clear and consistent. Structured maintenance schedules, documented safety inspections, asset tracking, and accessible compliance reports give teams the proof auditors expect without adding extra systems.
TMA Systems supports that foundation with a flexible CMMS platform that adapts to diverse operational realities.
- WebTMA supports enterprise teams managing complex facilities portfolios inside an enterprise CMMS environment.
- MEX CMMS serves manufacturing and industrial environments that depend on preventive maintenance, asset condition monitoring, and technician workflows aligned with ISO standards.
- EQ2 HEMS CMMS supports healthcare teams responsible for medical devices, FDA regulations, and digital signatures tied to regulatory documentation.
Each CMMS platform applies the same compliance principles while tailoring them to the environment's needs.
As compliance requirements grow more complex, teams can extend that CMMS foundation without disruption. Virtual Facility adds alarm monitoring and documented alert response to strengthen safety and audit accountability, while ProCal delivers calibration management for regulated instruments that require traceability, tolerance validation, and digital records.
Together, these solutions apply consistent compliance principles while scaling to meet industry-specific audit expectations.
FAQs about CMMS compliance
- CMMS compliance turns daily maintenance activity into audit-ready proof under regulatory review.
- Regulated industries adopt CMMS to control risk, document work, and protect teams during inspections.
- Structured records, inspections, and reporting make compliance repeatable rather than reactive.

Download the eBook now
You’re all set!
Your eBook is on its way to your inbox. We hope it brings fresh insights and practical takeaways to help you get more from your maintenance operations.
Explore related resources
.avif)
.svg)
This guide explains how CMMS enables compliance by industry. Explore features to evaluate and best practices for audit readiness in 2026.
Compliance now carries real operational and career risk for facility, maintenance, and compliance leaders. Missed inspections, undocumented repairs, and scattered records surface fast during audits, placing teams under pressure to defend decisions made months earlier.
As regulatory scrutiny increases across healthcare, manufacturing, food processing, energy, and other critical industries, accountability no longer stops at completing the work itself. Auditors expect proof that work followed defined standards every time.
Industry research backs this shift. Regulatory compliance is a major factor driving CMMS (Computerized Maintenance Management Software) adoption in the U.S., especially in healthcare, pharmaceuticals, food processing, and energy industries (Grand View Research). Teams no longer adopt systems for convenience. They adopt them to protect operations when regulators arrive.
A well-implemented CMMS platform gives teams the structure to treat compliance as part of daily operations rather than a scramble before inspections:
- Preventive maintenance schedules document required tasks. Inspections capture safety and quality checks in real time.
- Work orders connect issues to corrective actions.
- Reporting pulls that history into audit-ready records that stand up under review.
That foundation makes compliance repeatable, provable, and defensible across every facility, asset, and audit cycle.
What CMMS compliance really means for facilities and maintenance teams
CMMS compliance means using a maintenance management system to consistently document, track, and prove that required maintenance, inspections, and corrective actions were completed according to defined standards. During audits, regulatory bodies assess how well teams follow those standards in daily operations, not how quickly they react once problems surface. Clear, complete records demonstrate how work progressed from discovery to resolution across the full asset lifecycle.
Compliance does not stop at task completion. A CMMS must preserve when work occurred, who approved it, and how it aligned with standard operating procedures so teams can confidently defend maintenance decisions during regulatory reviews.
Core CMMS capabilities make that defensibility possible on a daily basis:
- Compliance tracking and asset records capture asset data, conditions, approvals, and changes at every step.
- Preventive and predictive maintenance programs structure recurring work and surface risk before failures disrupt operations.
- Work order management workflows ensure inspection findings and issues are always linked to documented corrective actions.
- Reporting and dashboards consolidate maintenance and inspection data into audit-ready compliance reports without manual reconciliation.
Audit documents teams pull first
Most audits begin with a short list of records. Maintenance teams usually need preventive maintenance history for regulated equipment, lists of overdue inspections, corrective action logs tied to recent findings, and asset condition reports that reflect how maintenance decisions align with regulatory standards. A CMMS that produces these compliance reports in minutes shortens every audit conversation.
Each audit record auditors request is produced by a specific CMMS capability. The table below connects those capabilities to compliance outcomes.
CMMS compliance features buyers should evaluate
Not all CMMS platforms handle compliance the same way. Focus on the features that shape audit readiness and reduce compliance exposure at scale.
- Audit-ready reporting: Pull filtered records for a defined asset class across a selected date range, then export compliance reports when regulatory bodies request maintenance history.
- Role-based access control: Restrict editing rights, track approvals, and protect asset data so audit trails reflect real accountability.
- Workflow configurability: Align inspection, maintenance, and corrective action workflows to regulatory requirements and standard operating procedures.
- Multi-site consistency: Apply the same maintenance management and inspection standards across locations while preserving local accountability.
- Asset history and traceability: Retain long-term records that connect findings, actions, asset conditions, and outcomes for regulated equipment.
- Integration readiness: Connect the CMMS to monitoring, calibration, or predictive maintenance systems that support regulated workflows and deliver real-time insights.
Facilities teams usually compare platforms based on how well each system protects them during audits, once regulatory pressure comes into play. The best CMMS software stands out when compliance remains intact even as operations scale.
CMMS compliance by industry
Compliance requirements look different depending on what teams manage and who audits their work. A strong CMMS adapts to each regulatory environment while relying on a common maintenance foundation across all facilities.
Healthcare teams using a healthcare CMMS track patient safety checks, equipment maintenance, and corrective actions required for accreditation. Districts and campus leaders working with a CMMS for education centralize inspections and asset history across aging buildings. Manufacturing, food production, and energy operations apply the same structure to meet safety, sanitation, and environmental standards.
The table below highlights how compliance expectations differ by industry and how a CMMS supports audit readiness across each regulatory environment.
When to expand beyond CMMS for compliance
Many organizations meet compliance obligations with a well-implemented CMMS that supports asset tracking, safety inspections, and audit reporting. Operations with growing asset portfolios, multi-site complexity, or tighter regulatory scrutiny often need added systems that extend compliance coverage without replacing the CMMS foundation.
Enterprise asset management
Large organizations manage layered asset hierarchies, capital plans, and multi-year audit histories tied to asset lifecycle performance. Enterprise asset management software extends asset tracking across facilities and supports long-term decision-making informed by manufacturer guidelines, ISO standards, and internal governance requirements.
Teams evaluating CMMS vs EAM, or reviewing the best enterprise asset management software, often reach this stage after compliance reviews expose gaps tied to scale rather than execution.
Alarm monitoring
High-risk environments require documented responses to safety and operational alerts tied to safety inspections. Alarm monitoring software connects real-time system events to maintenance actions so every alert produces a tracked work order and audit trail. That visibility protects teams when regulatory bodies review how issues surfaced and how fast corrective action followed.
Calibration management
Regulated instruments demand proof of accuracy, tolerance, and traceability. In healthcare and life sciences, FDA regulations governing medical devices often require calibration evidence beyond standard maintenance logs.
Calibration management software captures schedules, results, digital signatures, and historical records tied to manufacturer guidelines. Without dedicated calibration records, CMMS data leaves quality auditors with unanswered questions.
CMMS best practices to support ongoing compliance
Getting compliance-ready requires disciplined daily processes supported through maintenance management software rather than last-minute reporting.
- Standardize preventive maintenance schedules: Assign PM intervals at the asset level and review overdue rates weekly. Rising overdue counts often surface before regulatory agencies flag a compliance violation.
- Use inspection templates tied to safety protocols. Track inspection completion rates each month and align forms with the regulatory frameworks that govern your industry. Gaps in completion data often signal exposure during regulatory reviews.
- Link every issue to a corrective work order: Require work orders for all inspection findings and safety issues so electronic records show how problems moved from discovery to resolution.
- Review compliance dashboards routinely: Scan open inspections, late PMs, and missing documentation each week. Real-time visibility shortens response time when regulators request proof.
- Protect data integrity with role controls: Restrict editing rights, require digital signature approval for regulated actions, and preserve audit trails inside electronic records. Clean documentation protects teams when compliance questions surface.
Bringing it together: A practical approach to CMMS compliance
Many organizations meet their compliance obligations with a well-implemented CMMS when requirements remain clear and consistent. Structured maintenance schedules, documented safety inspections, asset tracking, and accessible compliance reports give teams the proof auditors expect without adding extra systems.
TMA Systems supports that foundation with a flexible CMMS platform that adapts to diverse operational realities.
- WebTMA supports enterprise teams managing complex facilities portfolios inside an enterprise CMMS environment.
- MEX CMMS serves manufacturing and industrial environments that depend on preventive maintenance, asset condition monitoring, and technician workflows aligned with ISO standards.
- EQ2 HEMS CMMS supports healthcare teams responsible for medical devices, FDA regulations, and digital signatures tied to regulatory documentation.
Each CMMS platform applies the same compliance principles while tailoring them to the environment's needs.
As compliance requirements grow more complex, teams can extend that CMMS foundation without disruption. Virtual Facility adds alarm monitoring and documented alert response to strengthen safety and audit accountability, while ProCal delivers calibration management for regulated instruments that require traceability, tolerance validation, and digital records.
Together, these solutions apply consistent compliance principles while scaling to meet industry-specific audit expectations.
FAQs about CMMS compliance
- CMMS compliance turns daily maintenance activity into audit-ready proof under regulatory review.
- Regulated industries adopt CMMS to control risk, document work, and protect teams during inspections.
- Structured records, inspections, and reporting make compliance repeatable rather than reactive.

Register for your free webinar
You’re all set!
Your webinar is on its way to your inbox. We hope it brings fresh insights and practical takeaways to help you get more from your maintenance operations.
Explore related resources
.avif)
.svg)

Compliance now carries real operational and career risk for facility, maintenance, and compliance leaders. Missed inspections, undocumented repairs, and scattered records surface fast during audits, placing teams under pressure to defend decisions made months earlier.
As regulatory scrutiny increases across healthcare, manufacturing, food processing, energy, and other critical industries, accountability no longer stops at completing the work itself. Auditors expect proof that work followed defined standards every time.
Industry research backs this shift. Regulatory compliance is a major factor driving CMMS (Computerized Maintenance Management Software) adoption in the U.S., especially in healthcare, pharmaceuticals, food processing, and energy industries (Grand View Research). Teams no longer adopt systems for convenience. They adopt them to protect operations when regulators arrive.
A well-implemented CMMS platform gives teams the structure to treat compliance as part of daily operations rather than a scramble before inspections:
- Preventive maintenance schedules document required tasks. Inspections capture safety and quality checks in real time.
- Work orders connect issues to corrective actions.
- Reporting pulls that history into audit-ready records that stand up under review.
That foundation makes compliance repeatable, provable, and defensible across every facility, asset, and audit cycle.
What CMMS compliance really means for facilities and maintenance teams
CMMS compliance means using a maintenance management system to consistently document, track, and prove that required maintenance, inspections, and corrective actions were completed according to defined standards. During audits, regulatory bodies assess how well teams follow those standards in daily operations, not how quickly they react once problems surface. Clear, complete records demonstrate how work progressed from discovery to resolution across the full asset lifecycle.
Compliance does not stop at task completion. A CMMS must preserve when work occurred, who approved it, and how it aligned with standard operating procedures so teams can confidently defend maintenance decisions during regulatory reviews.
Core CMMS capabilities make that defensibility possible on a daily basis:
- Compliance tracking and asset records capture asset data, conditions, approvals, and changes at every step.
- Preventive and predictive maintenance programs structure recurring work and surface risk before failures disrupt operations.
- Work order management workflows ensure inspection findings and issues are always linked to documented corrective actions.
- Reporting and dashboards consolidate maintenance and inspection data into audit-ready compliance reports without manual reconciliation.
Audit documents teams pull first
Most audits begin with a short list of records. Maintenance teams usually need preventive maintenance history for regulated equipment, lists of overdue inspections, corrective action logs tied to recent findings, and asset condition reports that reflect how maintenance decisions align with regulatory standards. A CMMS that produces these compliance reports in minutes shortens every audit conversation.
Each audit record auditors request is produced by a specific CMMS capability. The table below connects those capabilities to compliance outcomes.
CMMS compliance features buyers should evaluate
Not all CMMS platforms handle compliance the same way. Focus on the features that shape audit readiness and reduce compliance exposure at scale.
- Audit-ready reporting: Pull filtered records for a defined asset class across a selected date range, then export compliance reports when regulatory bodies request maintenance history.
- Role-based access control: Restrict editing rights, track approvals, and protect asset data so audit trails reflect real accountability.
- Workflow configurability: Align inspection, maintenance, and corrective action workflows to regulatory requirements and standard operating procedures.
- Multi-site consistency: Apply the same maintenance management and inspection standards across locations while preserving local accountability.
- Asset history and traceability: Retain long-term records that connect findings, actions, asset conditions, and outcomes for regulated equipment.
- Integration readiness: Connect the CMMS to monitoring, calibration, or predictive maintenance systems that support regulated workflows and deliver real-time insights.
Facilities teams usually compare platforms based on how well each system protects them during audits, once regulatory pressure comes into play. The best CMMS software stands out when compliance remains intact even as operations scale.
CMMS compliance by industry
Compliance requirements look different depending on what teams manage and who audits their work. A strong CMMS adapts to each regulatory environment while relying on a common maintenance foundation across all facilities.
Healthcare teams using a healthcare CMMS track patient safety checks, equipment maintenance, and corrective actions required for accreditation. Districts and campus leaders working with a CMMS for education centralize inspections and asset history across aging buildings. Manufacturing, food production, and energy operations apply the same structure to meet safety, sanitation, and environmental standards.
The table below highlights how compliance expectations differ by industry and how a CMMS supports audit readiness across each regulatory environment.
When to expand beyond CMMS for compliance
Many organizations meet compliance obligations with a well-implemented CMMS that supports asset tracking, safety inspections, and audit reporting. Operations with growing asset portfolios, multi-site complexity, or tighter regulatory scrutiny often need added systems that extend compliance coverage without replacing the CMMS foundation.
Enterprise asset management
Large organizations manage layered asset hierarchies, capital plans, and multi-year audit histories tied to asset lifecycle performance. Enterprise asset management software extends asset tracking across facilities and supports long-term decision-making informed by manufacturer guidelines, ISO standards, and internal governance requirements.
Teams evaluating CMMS vs EAM, or reviewing the best enterprise asset management software, often reach this stage after compliance reviews expose gaps tied to scale rather than execution.
Alarm monitoring
High-risk environments require documented responses to safety and operational alerts tied to safety inspections. Alarm monitoring software connects real-time system events to maintenance actions so every alert produces a tracked work order and audit trail. That visibility protects teams when regulatory bodies review how issues surfaced and how fast corrective action followed.
Calibration management
Regulated instruments demand proof of accuracy, tolerance, and traceability. In healthcare and life sciences, FDA regulations governing medical devices often require calibration evidence beyond standard maintenance logs.
Calibration management software captures schedules, results, digital signatures, and historical records tied to manufacturer guidelines. Without dedicated calibration records, CMMS data leaves quality auditors with unanswered questions.
CMMS best practices to support ongoing compliance
Getting compliance-ready requires disciplined daily processes supported through maintenance management software rather than last-minute reporting.
- Standardize preventive maintenance schedules: Assign PM intervals at the asset level and review overdue rates weekly. Rising overdue counts often surface before regulatory agencies flag a compliance violation.
- Use inspection templates tied to safety protocols. Track inspection completion rates each month and align forms with the regulatory frameworks that govern your industry. Gaps in completion data often signal exposure during regulatory reviews.
- Link every issue to a corrective work order: Require work orders for all inspection findings and safety issues so electronic records show how problems moved from discovery to resolution.
- Review compliance dashboards routinely: Scan open inspections, late PMs, and missing documentation each week. Real-time visibility shortens response time when regulators request proof.
- Protect data integrity with role controls: Restrict editing rights, require digital signature approval for regulated actions, and preserve audit trails inside electronic records. Clean documentation protects teams when compliance questions surface.
Bringing it together: A practical approach to CMMS compliance
Many organizations meet their compliance obligations with a well-implemented CMMS when requirements remain clear and consistent. Structured maintenance schedules, documented safety inspections, asset tracking, and accessible compliance reports give teams the proof auditors expect without adding extra systems.
TMA Systems supports that foundation with a flexible CMMS platform that adapts to diverse operational realities.
- WebTMA supports enterprise teams managing complex facilities portfolios inside an enterprise CMMS environment.
- MEX CMMS serves manufacturing and industrial environments that depend on preventive maintenance, asset condition monitoring, and technician workflows aligned with ISO standards.
- EQ2 HEMS CMMS supports healthcare teams responsible for medical devices, FDA regulations, and digital signatures tied to regulatory documentation.
Each CMMS platform applies the same compliance principles while tailoring them to the environment's needs.
As compliance requirements grow more complex, teams can extend that CMMS foundation without disruption. Virtual Facility adds alarm monitoring and documented alert response to strengthen safety and audit accountability, while ProCal delivers calibration management for regulated instruments that require traceability, tolerance validation, and digital records.
Together, these solutions apply consistent compliance principles while scaling to meet industry-specific audit expectations.
FAQs about CMMS compliance


Related resources
Related resources
You’ve seen what’s possible—connect with us to learn how TMA Systems can support your goals beyond the event.
You’ve read the insights, now see how TMA Systems helps teams put them into practice.

From ideas to impact
You’ve read the insights, now see how TMA Systems helps teams put them into practice.

From screen to solution
You’ve watched what’s possible, now see how TMA Systems works for your organization.

From insight to implementation
You’ve explored the strategies, now see how we can bring them to life across your real facilities.

From insight to implementation
You’ve explored the strategies, now see how we can bring them to life across your real facilities.





.svg)


.avif)