Medical equipment maintenance software for Biomed
In this post
%20(1).avif)
1
2
3
Learn what medical equipment maintenance software does, who uses it, and how to choose the right biomed CMMS to improve safety and efficiency.
Biomedical teams carry a heavy load. They manage regulated medical devices and keep maintenance activity accurate, traceable, and ready for inspection at any moment. Manual spreadsheets and generic tools create gaps that cause delays, missed tasks, and inconsistent documentation.
Hospitals face growing pressure to keep equipment in service longer, meet regulatory expectations with fewer disruptions, and manage larger device inventories across clinical areas. According to Grand View Research, healthcare maintenance software adoption continues to rise as hospitals prioritize stronger preventive maintenance, reduced downtime, and reliable audit support.
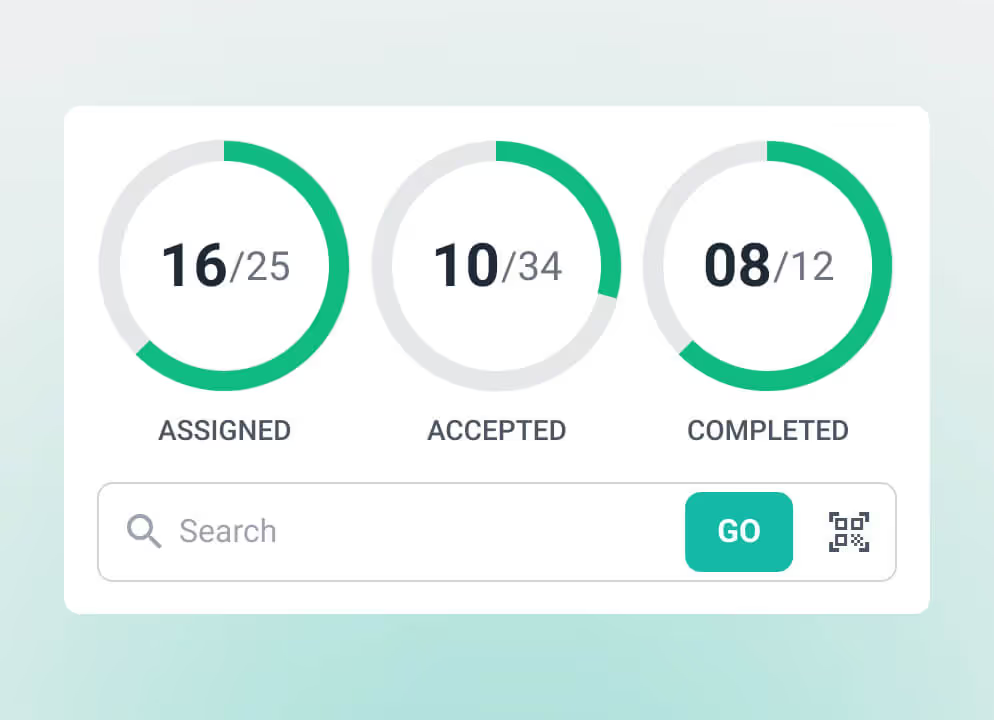
Digitized workflows give clinical engineering teams clearer visibility into device condition, testing schedules, risk, and service history. This improves uptime, strengthens inspection readiness, and reduces administrative overhead. A dedicated biomedical CMMS (Computerized Maintenance Management System) organizes maintenance, documentation, and reporting in one place, helping teams track required tasks and respond quickly during daily work.
TMA Systems supports this work with tools built for clinical engineering. Biomedical teams gain a stable foundation for managing device activity, maintaining accurate records, and staying ahead of equipment failures that impact patient care.
What is medical equipment maintenance software?
Medical equipment maintenance software helps biomedical teams plan, complete, and document work on regulated medical devices. It centralizes maintenance activity, tracks device history, and produces records that stay consistent and ready for inspection. The system replaces manual tools with workflows designed for clinical engineering, where accuracy and traceability are essential.
Core functions include:
Preventive maintenance scheduling
A scheduling engine organizes recurring tasks, testing intervals, and procedures based on manufacturer guidance and internal policies. Clear schedules and automated reminders help teams reduce missed preventive activities through preventive maintenance software designed for healthcare.
Corrective maintenance and repair tracking
Maintenance teams log repairs, labor time, parts, and follow-up actions in one system. This builds a reliable record for troubleshooting, spotting failure patterns, and understanding downtime events.
Device history and documentation
Each device carries a complete digital file, including PM activity, repairs, testing results, risk ratings, and asset details. This helps teams respond quickly during inspections and supports informed capital planning.
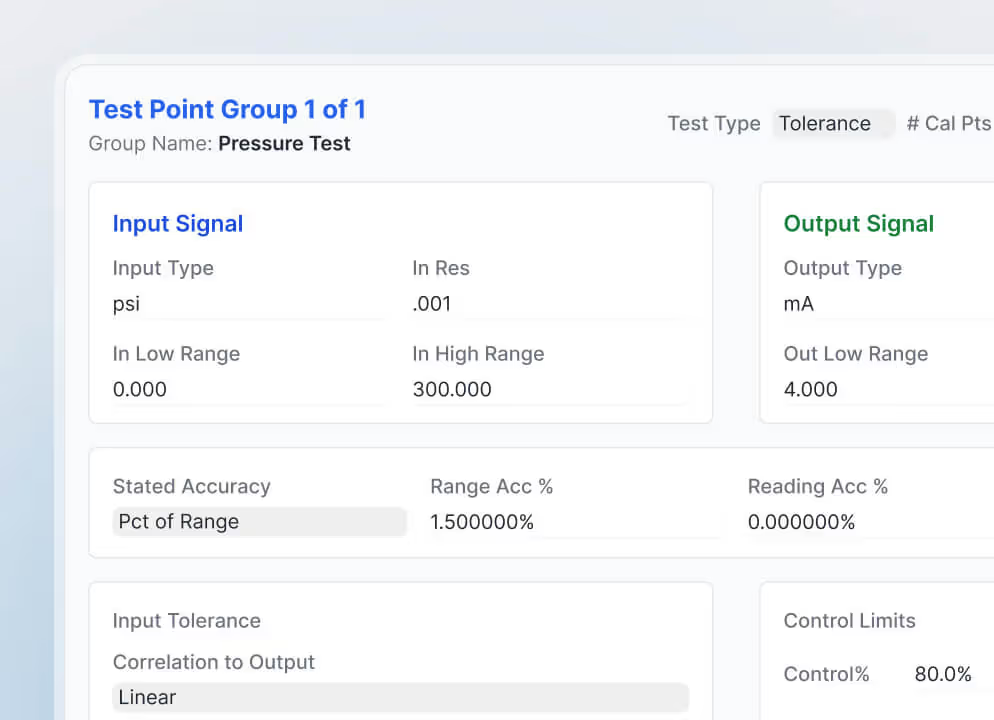
Calibration and safety testing management
Calibration work, electrical safety tests, and other regulated checks stay tied to individual devices and their required intervals. Centralized tracking improves accuracy and keeps clinical engineering aligned with testing schedules.
Recall and safety alert management
The system highlights affected devices and tracks corrective work through completion. Clear documentation reduces manual effort and protects device safety during recall events.
Mobile access for technicians
Technicians access work orders, procedures, checklists, and device histories on carts or handheld devices. This supports faster updates and improves documentation quality at the point of service.
Types of medical equipment maintenance software
Biomedical work requires accurate testing records, clear service histories, and workflows aligned with Joint Commission, CMS, FDA, and manufacturer standards. Several software categories support different parts of hospital operations. Clinical engineering teams often use a dedicated medical device maintenance platform alongside broader systems that provide enterprise context.
1. Biomed CMMS
A biomed CMMS focuses on device-level maintenance and clinical engineering workflows. It supports preventive maintenance, calibrations, electrical safety tests, work orders, device histories, and compliance reporting. This CMMS system aligns with the documentation and testing standards required in healthcare and keeps devices inspection-ready.
2. Healthcare Enterprise Asset Management software
Healthcare organizations can use enterprise asset management software to manage assets across facilities, IT, and clinical departments. Leadership uses this enterprise layer to track lifecycle costs, equipment age, risk, and replacement needs. Clinical engineering depends on a biomed-focused system for regulated device work while using the enterprise asset management layer for broader planning and coordination.
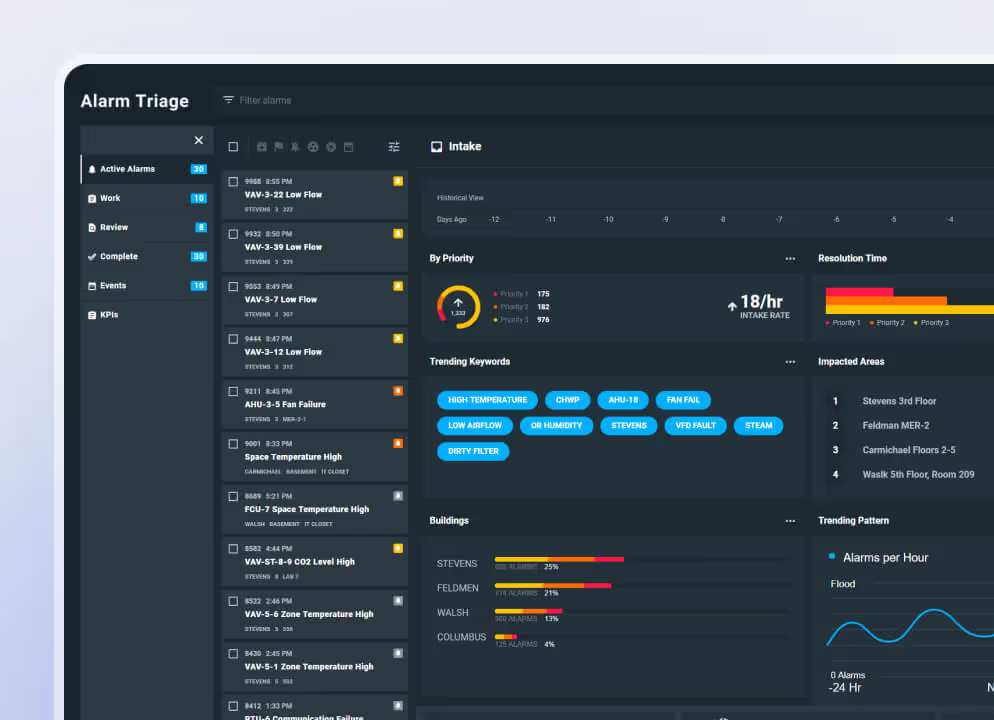
3. Alarm and Condition platforms
Hospitals use alarm management software to surface real-time equipment and environmental signals. These insights highlight alerts and conditions that indicate risk. Biomedical teams use this information to prioritize work and act before failures interrupt patient care. These platforms strengthen situational awareness but do not handle maintenance tasks or documentation.
Who uses medical equipment maintenance software?
Medical equipment maintenance software supports every level of clinical engineering. Biomedical technicians, clinical engineers, and clinical engineering directors use the system daily to complete maintenance, document regulated tasks, and prepare for inspections. They rely on accurate work order management software, device histories, and testing records to work efficiently.
A wider group across the hospital depends on this data. Compliance and risk teams review documented testing, repairs, and service logs. IT and cybersecurity teams use the device inventory to monitor connected equipment. Nursing and clinical staff report issues and check device status to avoid delays at the point of care. Operations and finance leaders use maintenance reporting and analytics to guide capital planning and replacement decisions. These teams access device safety, downtime risk, and regulatory activity through compliance tracking software.
A strong platform supports daily activity and long-term decisions involving safety, staffing, and equipment lifecycles.
How to evaluate medical equipment maintenance software
Medical equipment maintenance software shapes how clinical engineering teams manage regulated activity. These criteria highlight the features that matter most.
1. Regulatory and audit support
Look for software that produces trustworthy, inspection-ready records for preventive maintenance, calibrations, repairs, and testing. Accurate reporting helps teams respond quickly during surveys.
2. Depth of device-level workflows
Review how well the platform supports clinical engineering tasks. Workflows should match maintenance, calibration, electrical safety testing, and documentation needs without extra steps.
3. Accurate device inventory
A reliable system maintains current locations, statuses, risk levels, and asset details. Clean data supports daily work and long-term decision-making.
4. Handling of recalls and safety alerts
A reliable system maintains current locations, statuses, risk levels, and asset details. Clean data supports daily work and long-term decision-making.
5. Ease of use for technicians
Technicians need simple screens and fast navigation. Straightforward workflows reduce errors and support faster maintenance.
6. Mobile access at the point of service
Technicians should be able to view procedures, histories, and checklists on carts or handheld devices. Mobile access improves accuracy and speeds up documentation.
How TMA Systems supports biomedical asset management
TMA Systems provides an ecosystem built for regulated medical devices and the broader needs of healthcare operations. EQ2 HEMS and its integrated platforms support clinical engineering from routine service tasks to survey preparation and capital planning.
EQ2 HEMS
EQ2 HEMS is purpose-built for maintaining medical devices. The system manages preventive maintenance, calibrations, electrical safety tests, device histories, recalls, and compliance reporting aligned with Joint Commission, CMS, FDA, and manufacturer standards. Clinical engineering uses it to document work accurately and keep testing schedules current throughout the year.
WebTMA
WebTMA connects clinical engineering with facilities, IT, and operations through enterprise asset management. This broader view strengthens lifecycle planning, risk identification, and cross-department coordination.
Virtual Facility
Virtual Facility works alongside your biomed CMMS, surfacing real-time alarms, conditions, and risk indicators from the hospital environment. Built to extend platforms like EQ2 HEMS, Virtual Facility turns CMMS data and live signals into prioritized actions so biomed teams can act on emerging problems before they disrupt patient care.
ProCal
ProCal builds on the CMMS foundation for organizations with advanced calibration requirements. It adds deeper tracking, scheduling, and documentation for equipment that requires strict calibration procedures, while keeping all activity tied back to the core CMMS record.
Ultimately, these platforms come together to give healthcare teams a complete maintenance foundation.
EQ2 HEMS leads clinical engineering workflows, while WebTMA manages facility and operational assets. Virtual Facility and ProCal work as CMMS-dependent extensions, adding real-time alarm insight and precise calibration control. Together, they can form a connected ecosystem that supports daily maintenance, regulatory demands, and long-term planning.
FAQs about medical equipment maintenance software for biomed
- Biomed teams rely on medical equipment maintenance software to keep devices safe, compliant, and ready for patient care.
- Purpose-built biomed CMMS systems improve uptime, documentation accuracy, and survey readiness.
- A connected biomedical ecosystem strengthens safety, maintenance workflows, and lifecycle planning across the hospital.

Download the eBook now
You’re all set!
Your eBook is on its way to your inbox. We hope it brings fresh insights and practical takeaways to help you get more from your maintenance operations.
Explore related resources
.avif)
.svg)
Learn what medical equipment maintenance software does, who uses it, and how to choose the right biomed CMMS to improve safety and efficiency.
Biomedical teams carry a heavy load. They manage regulated medical devices and keep maintenance activity accurate, traceable, and ready for inspection at any moment. Manual spreadsheets and generic tools create gaps that cause delays, missed tasks, and inconsistent documentation.
Hospitals face growing pressure to keep equipment in service longer, meet regulatory expectations with fewer disruptions, and manage larger device inventories across clinical areas. According to Grand View Research, healthcare maintenance software adoption continues to rise as hospitals prioritize stronger preventive maintenance, reduced downtime, and reliable audit support.
Digitized workflows give clinical engineering teams clearer visibility into device condition, testing schedules, risk, and service history. This improves uptime, strengthens inspection readiness, and reduces administrative overhead. A dedicated biomedical CMMS (Computerized Maintenance Management System) organizes maintenance, documentation, and reporting in one place, helping teams track required tasks and respond quickly during daily work.
TMA Systems supports this work with tools built for clinical engineering. Biomedical teams gain a stable foundation for managing device activity, maintaining accurate records, and staying ahead of equipment failures that impact patient care.
What is medical equipment maintenance software?
Medical equipment maintenance software helps biomedical teams plan, complete, and document work on regulated medical devices. It centralizes maintenance activity, tracks device history, and produces records that stay consistent and ready for inspection. The system replaces manual tools with workflows designed for clinical engineering, where accuracy and traceability are essential.
Core functions include:
Preventive maintenance scheduling
A scheduling engine organizes recurring tasks, testing intervals, and procedures based on manufacturer guidance and internal policies. Clear schedules and automated reminders help teams reduce missed preventive activities through preventive maintenance software designed for healthcare.
Corrective maintenance and repair tracking
Maintenance teams log repairs, labor time, parts, and follow-up actions in one system. This builds a reliable record for troubleshooting, spotting failure patterns, and understanding downtime events.
Device history and documentation
Each device carries a complete digital file, including PM activity, repairs, testing results, risk ratings, and asset details. This helps teams respond quickly during inspections and supports informed capital planning.
Calibration and safety testing management
Calibration work, electrical safety tests, and other regulated checks stay tied to individual devices and their required intervals. Centralized tracking improves accuracy and keeps clinical engineering aligned with testing schedules.
Recall and safety alert management
The system highlights affected devices and tracks corrective work through completion. Clear documentation reduces manual effort and protects device safety during recall events.
Mobile access for technicians
Technicians access work orders, procedures, checklists, and device histories on carts or handheld devices. This supports faster updates and improves documentation quality at the point of service.
Types of medical equipment maintenance software
Biomedical work requires accurate testing records, clear service histories, and workflows aligned with Joint Commission, CMS, FDA, and manufacturer standards. Several software categories support different parts of hospital operations. Clinical engineering teams often use a dedicated medical device maintenance platform alongside broader systems that provide enterprise context.
1. Biomed CMMS
A biomed CMMS focuses on device-level maintenance and clinical engineering workflows. It supports preventive maintenance, calibrations, electrical safety tests, work orders, device histories, and compliance reporting. This CMMS system aligns with the documentation and testing standards required in healthcare and keeps devices inspection-ready.
2. Healthcare Enterprise Asset Management software
Healthcare organizations can use enterprise asset management software to manage assets across facilities, IT, and clinical departments. Leadership uses this enterprise layer to track lifecycle costs, equipment age, risk, and replacement needs. Clinical engineering depends on a biomed-focused system for regulated device work while using the enterprise asset management layer for broader planning and coordination.
3. Alarm and Condition platforms
Hospitals use alarm management software to surface real-time equipment and environmental signals. These insights highlight alerts and conditions that indicate risk. Biomedical teams use this information to prioritize work and act before failures interrupt patient care. These platforms strengthen situational awareness but do not handle maintenance tasks or documentation.
Who uses medical equipment maintenance software?
Medical equipment maintenance software supports every level of clinical engineering. Biomedical technicians, clinical engineers, and clinical engineering directors use the system daily to complete maintenance, document regulated tasks, and prepare for inspections. They rely on accurate work order management software, device histories, and testing records to work efficiently.
A wider group across the hospital depends on this data. Compliance and risk teams review documented testing, repairs, and service logs. IT and cybersecurity teams use the device inventory to monitor connected equipment. Nursing and clinical staff report issues and check device status to avoid delays at the point of care. Operations and finance leaders use maintenance reporting and analytics to guide capital planning and replacement decisions. These teams access device safety, downtime risk, and regulatory activity through compliance tracking software.
A strong platform supports daily activity and long-term decisions involving safety, staffing, and equipment lifecycles.
How to evaluate medical equipment maintenance software
Medical equipment maintenance software shapes how clinical engineering teams manage regulated activity. These criteria highlight the features that matter most.
1. Regulatory and audit support
Look for software that produces trustworthy, inspection-ready records for preventive maintenance, calibrations, repairs, and testing. Accurate reporting helps teams respond quickly during surveys.
2. Depth of device-level workflows
Review how well the platform supports clinical engineering tasks. Workflows should match maintenance, calibration, electrical safety testing, and documentation needs without extra steps.
3. Accurate device inventory
A reliable system maintains current locations, statuses, risk levels, and asset details. Clean data supports daily work and long-term decision-making.
4. Handling of recalls and safety alerts
A reliable system maintains current locations, statuses, risk levels, and asset details. Clean data supports daily work and long-term decision-making.
5. Ease of use for technicians
Technicians need simple screens and fast navigation. Straightforward workflows reduce errors and support faster maintenance.
6. Mobile access at the point of service
Technicians should be able to view procedures, histories, and checklists on carts or handheld devices. Mobile access improves accuracy and speeds up documentation.
How TMA Systems supports biomedical asset management
TMA Systems provides an ecosystem built for regulated medical devices and the broader needs of healthcare operations. EQ2 HEMS and its integrated platforms support clinical engineering from routine service tasks to survey preparation and capital planning.
EQ2 HEMS
EQ2 HEMS is purpose-built for maintaining medical devices. The system manages preventive maintenance, calibrations, electrical safety tests, device histories, recalls, and compliance reporting aligned with Joint Commission, CMS, FDA, and manufacturer standards. Clinical engineering uses it to document work accurately and keep testing schedules current throughout the year.
WebTMA
WebTMA connects clinical engineering with facilities, IT, and operations through enterprise asset management. This broader view strengthens lifecycle planning, risk identification, and cross-department coordination.
Virtual Facility
Virtual Facility works alongside your biomed CMMS, surfacing real-time alarms, conditions, and risk indicators from the hospital environment. Built to extend platforms like EQ2 HEMS, Virtual Facility turns CMMS data and live signals into prioritized actions so biomed teams can act on emerging problems before they disrupt patient care.
ProCal
ProCal builds on the CMMS foundation for organizations with advanced calibration requirements. It adds deeper tracking, scheduling, and documentation for equipment that requires strict calibration procedures, while keeping all activity tied back to the core CMMS record.
Ultimately, these platforms come together to give healthcare teams a complete maintenance foundation.
EQ2 HEMS leads clinical engineering workflows, while WebTMA manages facility and operational assets. Virtual Facility and ProCal work as CMMS-dependent extensions, adding real-time alarm insight and precise calibration control. Together, they can form a connected ecosystem that supports daily maintenance, regulatory demands, and long-term planning.
FAQs about medical equipment maintenance software for biomed
- Biomed teams rely on medical equipment maintenance software to keep devices safe, compliant, and ready for patient care.
- Purpose-built biomed CMMS systems improve uptime, documentation accuracy, and survey readiness.
- A connected biomedical ecosystem strengthens safety, maintenance workflows, and lifecycle planning across the hospital.

Register for your free webinar
You’re all set!
Your webinar is on its way to your inbox. We hope it brings fresh insights and practical takeaways to help you get more from your maintenance operations.
Explore related resources
.avif)
.svg)

Biomedical teams carry a heavy load. They manage regulated medical devices and keep maintenance activity accurate, traceable, and ready for inspection at any moment. Manual spreadsheets and generic tools create gaps that cause delays, missed tasks, and inconsistent documentation.
Hospitals face growing pressure to keep equipment in service longer, meet regulatory expectations with fewer disruptions, and manage larger device inventories across clinical areas. According to Grand View Research, healthcare maintenance software adoption continues to rise as hospitals prioritize stronger preventive maintenance, reduced downtime, and reliable audit support.
Digitized workflows give clinical engineering teams clearer visibility into device condition, testing schedules, risk, and service history. This improves uptime, strengthens inspection readiness, and reduces administrative overhead. A dedicated biomedical CMMS (Computerized Maintenance Management System) organizes maintenance, documentation, and reporting in one place, helping teams track required tasks and respond quickly during daily work.
TMA Systems supports this work with tools built for clinical engineering. Biomedical teams gain a stable foundation for managing device activity, maintaining accurate records, and staying ahead of equipment failures that impact patient care.
What is medical equipment maintenance software?
Medical equipment maintenance software helps biomedical teams plan, complete, and document work on regulated medical devices. It centralizes maintenance activity, tracks device history, and produces records that stay consistent and ready for inspection. The system replaces manual tools with workflows designed for clinical engineering, where accuracy and traceability are essential.
Core functions include:
Preventive maintenance scheduling
A scheduling engine organizes recurring tasks, testing intervals, and procedures based on manufacturer guidance and internal policies. Clear schedules and automated reminders help teams reduce missed preventive activities through preventive maintenance software designed for healthcare.
Corrective maintenance and repair tracking
Maintenance teams log repairs, labor time, parts, and follow-up actions in one system. This builds a reliable record for troubleshooting, spotting failure patterns, and understanding downtime events.
Device history and documentation
Each device carries a complete digital file, including PM activity, repairs, testing results, risk ratings, and asset details. This helps teams respond quickly during inspections and supports informed capital planning.
Calibration and safety testing management
Calibration work, electrical safety tests, and other regulated checks stay tied to individual devices and their required intervals. Centralized tracking improves accuracy and keeps clinical engineering aligned with testing schedules.
Recall and safety alert management
The system highlights affected devices and tracks corrective work through completion. Clear documentation reduces manual effort and protects device safety during recall events.
Mobile access for technicians
Technicians access work orders, procedures, checklists, and device histories on carts or handheld devices. This supports faster updates and improves documentation quality at the point of service.
Types of medical equipment maintenance software
Biomedical work requires accurate testing records, clear service histories, and workflows aligned with Joint Commission, CMS, FDA, and manufacturer standards. Several software categories support different parts of hospital operations. Clinical engineering teams often use a dedicated medical device maintenance platform alongside broader systems that provide enterprise context.
1. Biomed CMMS
A biomed CMMS focuses on device-level maintenance and clinical engineering workflows. It supports preventive maintenance, calibrations, electrical safety tests, work orders, device histories, and compliance reporting. This CMMS system aligns with the documentation and testing standards required in healthcare and keeps devices inspection-ready.
2. Healthcare Enterprise Asset Management software
Healthcare organizations can use enterprise asset management software to manage assets across facilities, IT, and clinical departments. Leadership uses this enterprise layer to track lifecycle costs, equipment age, risk, and replacement needs. Clinical engineering depends on a biomed-focused system for regulated device work while using the enterprise asset management layer for broader planning and coordination.
3. Alarm and Condition platforms
Hospitals use alarm management software to surface real-time equipment and environmental signals. These insights highlight alerts and conditions that indicate risk. Biomedical teams use this information to prioritize work and act before failures interrupt patient care. These platforms strengthen situational awareness but do not handle maintenance tasks or documentation.
Who uses medical equipment maintenance software?
Medical equipment maintenance software supports every level of clinical engineering. Biomedical technicians, clinical engineers, and clinical engineering directors use the system daily to complete maintenance, document regulated tasks, and prepare for inspections. They rely on accurate work order management software, device histories, and testing records to work efficiently.
A wider group across the hospital depends on this data. Compliance and risk teams review documented testing, repairs, and service logs. IT and cybersecurity teams use the device inventory to monitor connected equipment. Nursing and clinical staff report issues and check device status to avoid delays at the point of care. Operations and finance leaders use maintenance reporting and analytics to guide capital planning and replacement decisions. These teams access device safety, downtime risk, and regulatory activity through compliance tracking software.
A strong platform supports daily activity and long-term decisions involving safety, staffing, and equipment lifecycles.
How to evaluate medical equipment maintenance software
Medical equipment maintenance software shapes how clinical engineering teams manage regulated activity. These criteria highlight the features that matter most.
1. Regulatory and audit support
Look for software that produces trustworthy, inspection-ready records for preventive maintenance, calibrations, repairs, and testing. Accurate reporting helps teams respond quickly during surveys.
2. Depth of device-level workflows
Review how well the platform supports clinical engineering tasks. Workflows should match maintenance, calibration, electrical safety testing, and documentation needs without extra steps.
3. Accurate device inventory
A reliable system maintains current locations, statuses, risk levels, and asset details. Clean data supports daily work and long-term decision-making.
4. Handling of recalls and safety alerts
A reliable system maintains current locations, statuses, risk levels, and asset details. Clean data supports daily work and long-term decision-making.
5. Ease of use for technicians
Technicians need simple screens and fast navigation. Straightforward workflows reduce errors and support faster maintenance.
6. Mobile access at the point of service
Technicians should be able to view procedures, histories, and checklists on carts or handheld devices. Mobile access improves accuracy and speeds up documentation.
How TMA Systems supports biomedical asset management
TMA Systems provides an ecosystem built for regulated medical devices and the broader needs of healthcare operations. EQ2 HEMS and its integrated platforms support clinical engineering from routine service tasks to survey preparation and capital planning.
EQ2 HEMS
EQ2 HEMS is purpose-built for maintaining medical devices. The system manages preventive maintenance, calibrations, electrical safety tests, device histories, recalls, and compliance reporting aligned with Joint Commission, CMS, FDA, and manufacturer standards. Clinical engineering uses it to document work accurately and keep testing schedules current throughout the year.
WebTMA
WebTMA connects clinical engineering with facilities, IT, and operations through enterprise asset management. This broader view strengthens lifecycle planning, risk identification, and cross-department coordination.
Virtual Facility
Virtual Facility works alongside your biomed CMMS, surfacing real-time alarms, conditions, and risk indicators from the hospital environment. Built to extend platforms like EQ2 HEMS, Virtual Facility turns CMMS data and live signals into prioritized actions so biomed teams can act on emerging problems before they disrupt patient care.
ProCal
ProCal builds on the CMMS foundation for organizations with advanced calibration requirements. It adds deeper tracking, scheduling, and documentation for equipment that requires strict calibration procedures, while keeping all activity tied back to the core CMMS record.
Ultimately, these platforms come together to give healthcare teams a complete maintenance foundation.
EQ2 HEMS leads clinical engineering workflows, while WebTMA manages facility and operational assets. Virtual Facility and ProCal work as CMMS-dependent extensions, adding real-time alarm insight and precise calibration control. Together, they can form a connected ecosystem that supports daily maintenance, regulatory demands, and long-term planning.
FAQs about medical equipment maintenance software for biomed


Related resources
Related resources
You’ve seen what’s possible—connect with us to learn how TMA Systems can support your goals beyond the event.
You’ve read the insights, now see how TMA Systems helps teams put them into practice.


From ideas to impact
You’ve read the insights, now see how TMA Systems helps teams put them into practice.


From screen to solution
You’ve watched what’s possible, now see how TMA Systems works for your organization.


From insight to implementation
You’ve explored the strategies, now see how we can bring them to life across your real facilities.


From insight to implementation
You’ve explored the strategies, now see how we can bring them to life across your real facilities.








.avif)